![]()
Visiting standards during COVID-19
Four visitors are allowed at the hospital bedside, including parents/legal guardians. If a child age 5-17 is assigned as one of the visitors, they must be from the same household. Two adults can be with a child when coming for surgery. At this time, children under 5 may not visit. See the Welcome Center for a wellness screening.
Clinic visitor standards
We recommend bringing only the people who need to be at your child’s clinic visit. Please reschedule if you have symptoms of illness.
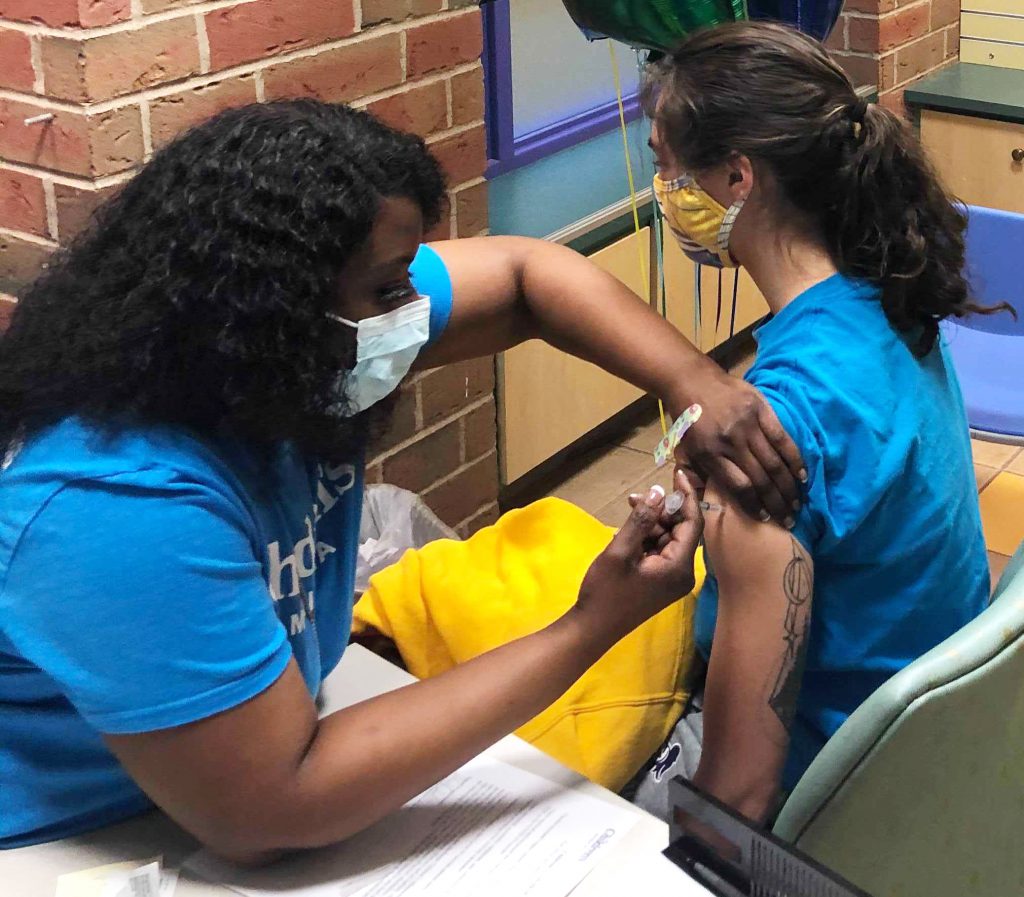
Vaccines, boosters and scheduling
The Centers for Disease Control and Prevention (CDC) gave final approval for kids 6 months and older to be vaccinated against COVID-19 under the Food and Drug Administration (FDA) Emergency Use Authorization.
COVID-19 vaccines: common questions and answers
Children’s Minnesota experts teamed up to provide you with answers to all your questions about the COVID-19 vaccine, safety concerns and side effects.
Learn more about Emergency Use Authorization (EUA)
Information fact sheets for recipients and caregivers considering the two-dose Pfizer-BioNTech vaccine.
For kids age 5-11, click here.
For kids age 12 and up, click here.
YouTube: COVID-19 vaccines and kids; What pediatricians are saying
The COVID-19 vaccine has been shown to be safe and effective for kids and teens. Talk with your child’s provider. They are there to help you.

COVID-19 medications for families
For kids with underlying medical conditions, these therapy options could be helpful in preventing severe COVID-19.
I think my child has COVID-19. What do I do now?
We urge you to stay home and call before coming to an emergency room or clinic, and know that we are ready to help if you do need to come in.
Testing
Find out the differences between tests, when you should get tested and where you can get tested. COVID-19 testing is available at all nine primary care clinics for children with or without symptoms. A provider visit is required with testing. Availability for COVID-19 testing, and the time it takes to get results, may vary.
Hotline
If your child is a patient of Children’s Minnesota (or a Children’s Health Network clinic), please call our nurse line below:
If not, please call the MDH hotline.
MDH Hotline
The MDH COVID-19 Public Hotline can help you locate testing sites and treatment sites (for oral COVID-19 therapies and monoclonal antibody treatment). If you don’t have a primary care provider and need help finding a clinic, please call the hotline for a list of low-cost clinics near you. People are available to speak to you in English, Hmong, Somali, Spanish, Karen and many other languages.
Hotline hours are Monday-Friday 9 a.m. to 7 p.m., Saturday 10 a.m. to 6 p.m.
My child needs emergency, primary, or other care.
Our pediatricians urge families to not wait to get care like critical vaccinations and treatment for injuries. Families may be worried about bringing their children to a hospital or clinic to receive care. But we want you to know that we’re here for you and have taken many extra steps so we can safely care for your child, including virtual care options.
Emergency rooms
Our emergency rooms are open and safe to come to. Do not delay emergency care, since doing so can be dangerous.
Surgery
Surgeries are still being scheduled. Our team is taking extra precautions to keep your family safe.
Specialty care
In-person and virtual visits are available at many clinics, and some have set hours to care only for kids who are feeling well.
Virtual care
Virtual visits are available at many of our clinics, and for a variety of appointment types.
Well-child check-ups
You can still bring your child in for well-child check-ups and immunizations, essential for the development of children.
Behavioral health
Virtual visits are available for many appointments, and in-person visits are available for other urgent care.